Chlorine dioxide: a better disinfection solution
Chlorine dioxide: a better disinfection solution
Choosing a disinfectant for both medical devices and surfaces can be confusing. With so many different chemistries, modes of action, contact times, delivery methods, OH&S profiles and microbiological efficacies on offer, how do you select the right product?
Chlorine dioxide (ClO2) has been used for decades as an effective disinfectant in a wide range of industries and is fast gaining recognition in the Australasian healthcare industry with an unparalleled reputation.
ClO2 outperforms alternative chemistries, providing superior disinfection that can be tailored for various applications. Effective in exceptionally short contact times at lower concentrations, chlorine dioxide demonstrates a good safety profile whilst maintaining efficacy.
This makes it an ideal chemistry for use in healthcare, where fast, effective and simple disinfection is paramount.

What is chlorine dioxide?
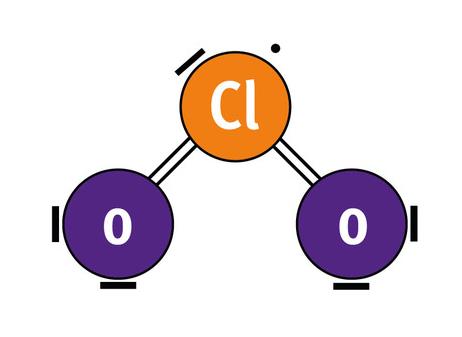
Chlorine dioxide is an unusual molecule with structural bonding once thought to be impossible. The chlorine atom requires more bonds that it can physically withstand, which causes one of the electrons to be in constant exchange between the two oxygen atoms. This electron exchange is called Resonance and is the key reason why this chemistry is so unique. This constant vibration is a causative for why the oxidising agent can boast a broad-spectrum efficacy including bacteria, yeast, fungi, viruses, mycobacteria, protozoa and bacterial spores. Furthermore, microorganisms cannot build resistance to chlorine dioxide.
Surface disinfection
Chlorine dioxide ismore effective than conventional chlorine solution, one of the most commonly used surface disinfectants worldwide. Other chemistries such as alcohols or quaternary ammonium compounds (QACs) can provide reasonable efficacy against bacteria and fungi, but they are far less effective against viruses and mycobacteria and have no credible efficacy against bacterial spores in acceptable contact times.
Many disinfectant chemistries will successfully pass efficacy tests when tested under specific parameters. These can include clean or dirty conditions, cold or warm temperatures, extended contact times and high volumes of disinfectant liquid, meaning the tests are not always representative of reallife scenarios. Chlorine dioxide is effective within realistic user parameters.

Device disinfection
Oxidising agents such as ClO2 are typically chosen for device disinfection due to their speed and broad-spectrum efficacy. However, substitute chemistries such as hydrogen peroxide and peracetic acid are known to be corrosive and potentially hazardous to clinical users. Medical devices have been successfully validated for disinfection using chlorine dioxide at ambient temperatures and low concentrations. Resulting in a safer user environment, and a longer life for the medical device itself.
Compatibility and safety
Chlorine dioxide is generated by combining base solution (citric acid) and activator solution (sodium chlorite) at point of use. This separation of solutions ensures that the chemistry is freshly generated and at its peak performance when applied to a medical surface. It also gives the product a long shelf life in comparison to other oxidisers. ClO2 is known to be compatible with materials commonly found in medical equipment and environments and has an excellent health and safety profile when used at recommended levels.
